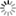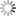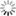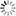Quantum Effect
- [Wave Functions of the Electron in a Hydrogen Atom - Wikipedia: Wave functions of the electron in a hydrogen atom at different energy levels. Quantum mechanics cannot predict the exact location of a particle in space, only the probability of finding it at different locations. The brighter areas represent a higher probability of finding the electron.]
- Overview
Quantum effects are not limited to the atomic scale. There are several examples of macroscopic quantum behavior. Quantum physics describes matter and energy as quantum wave functions, which are sometimes like waves and sometimes like particles, but are actually more complex entities than waves or particles.
Virtually every object in the universe, from atoms to stars, operates according to quantum physics. In many cases, such as when throwing a baseball, quantum physics leads to the same results as classical physics. In this case, we use classical physics rather than quantum physics because the math is easier and the principles are more intuitive. The laws of quantum physics still operate on the baseball field, but their operation is not obvious, so we say the system is non-quantum. When the quantum behavior of a situation becomes apparent, it is described as quantum, even though it is actually always quantum.
"Quantum Effects" are effects that classical physics cannot correctly predict but that quantum theory can correctly predict. Classical physics describes matter as being composed of small solid particles. So as long as we make fragments of matter behave like waves, we are exhibiting quantum effects. (Classical waves like sound waves and ocean waves don't count as quantum, because motion is waves, but fragments are still small solid spheres. In order for quantum effects to occur, the particles themselves must behave like waves.)
- Topics of Quantum Effect
- Discrete
- Quanta
- Particle and wave duality
- Probabilistic
- Uncertainty
- Superposition
- Schrödinger’s cat
- Cat state
- Entanglement
- Spooky action at a distance
- Bell’s theorem and Bell’s inequality
- Bell states
- Phase
- Interference
- Wave function
- Density matrix or density operator
- Probability amplitude
- Unitarity principle
- Basis state
- Computational basis state
- Quantum state
- State vector
- Collapse of wave function on measurement
- Measurement
- No-cloning theorem
- Hamiltonian
- Schrödinger’s equation
- Time evolution
- Fermi-Dirac statistics
- Bose-Einstein statistics
- Fermions
- Bosons
- Pauli exclusion principle
- Spin
- Integer spin
- Half-spin or half-integer spin or spin 1/2
- Spin up and spin down
- Cooper pairs
- Superconductivity and superfluidity
- Tunneling
- Josephson effect
- Quantum hall effect
- Macroscopic quantum effects
- Zero-point energy and vacuum fluctuations
- The Key Advantages of Quantum Information Science over Clasical Methods
Quantum information science has three key advantages over classical methods. All of these advantages benefit from the magic of quantum effects enabled by quantum mechanics.
- Quantum computing provides higher performance than classical computing by providing exponentially accelerated quantum parallelism - evaluating many (all) possibilities in parallel in a single computation.
- Quantum communication provides inherent security through quantum entanglement - also known as ghost operations at a distance, as opposed to security, which is a problematic afterthought for classical communication and networking.
- Quantum metrology and quantum sensing provide higher accuracy and precision for the measurement of physical quantities and the detection of objects.
- Quantum Mechanics
Quantum mechanics is a fundamental theory in physics that describes the physical properties of nature on the scale of atoms and subatomic particles. It is the foundation of all quantum physics, including quantum chemistry, quantum field theory, quantum technology, and quantum information science.
Classical physics is a collection of theories that existed before quantum mechanics, which describe many aspects of nature on the ordinary (macro) scale, but not enough to describe them on the small (atomic and subatomic) scale. Most theories in classical physics can be derived from quantum mechanics as approximations that work on large (macro) scales.
Quantum mechanics differs from classical physics in that the energy, momentum, angular momentum, and other quantities of bound systems are restricted to discrete values (quantization), objects have the properties of particles and waves (wave-particle duality), and Existing limits Given a complete set of initial conditions (uncertainty principle), the value of a physical quantity can be accurately predicted before measurement.
Quantum mechanics gradually emerged from theory to explain observations that were incompatible with classical physics, such as Max Planck's solution to the blackbody radiation problem in 1900, and Albert Einstein's explanation of the photoelectric effect in 1905 Correspondence between energy and frequency in the paper of Comprehensive development of quantum mechanics.
Modern theories are formulated in various specially developed mathematical forms. In one of them, a mathematical entity called a wavefunction provides information about measurements of particle energy, momentum and other physical properties in the form of probabilistic magnitudes.
[More to come ...]